Read More in The Sun Gro’er Issue 3/3 (2005)
Growing media pH is a perennial subject of interest in our industry. One reason is that unlike mineral soils where plants grow naturally, peat mixes are not well buffered against pH changes. Another reason is that, again, unlike natural conditions where plants can grow more roots in favorable pH zones, the limited volume of mix in containers exacerbates pH effects. Drastic pH changes upset plant quality (figure 1).
The grower’s water, fertilizer regime, and even the plant species being grown also affect pH. The growing medium is the first thing questioned when pH problems occur, because pH changes occur right in the medium.
Here, I present the latest research in the area of growing media pH. The information may fulfill your curiosity, your practical needs or just give your talking points about how the industry is striving to manage materials to produce a better growing media.
Truck-wide Variation
 Peat is acidic and lime is added to neutralize some of that acidity and raise pH, however, even when the same amount of lime is added to the same amount of peat every time, the resulting mix pH is not the same every time. The pH variation can be, as the famous remark goes, wide enough for the truck carrying the mix to go through from 5.0 to 7.0. Remember that pH scale is logarithmic: a pH of 5 is 10 times more acidic than pH of 6 and 100 times more acidic than 7. Therefore, the variation is wide. If your blood pH varies by just 0.2, problems occur in your body. Don’t worry, your body is so efficient, its buffer system kicks in and brings blood pH back to the original value in less than a minute. (Don’t you wish your mix maintained pH like blood in your body?) To build such a buffer system into a mix, one first needs to know what causes pH variation in mixes. So, we at Sun Gro explored this further in a quantitative manner.
Peat is acidic and lime is added to neutralize some of that acidity and raise pH, however, even when the same amount of lime is added to the same amount of peat every time, the resulting mix pH is not the same every time. The pH variation can be, as the famous remark goes, wide enough for the truck carrying the mix to go through from 5.0 to 7.0. Remember that pH scale is logarithmic: a pH of 5 is 10 times more acidic than pH of 6 and 100 times more acidic than 7. Therefore, the variation is wide. If your blood pH varies by just 0.2, problems occur in your body. Don’t worry, your body is so efficient, its buffer system kicks in and brings blood pH back to the original value in less than a minute. (Don’t you wish your mix maintained pH like blood in your body?) To build such a buffer system into a mix, one first needs to know what causes pH variation in mixes. So, we at Sun Gro explored this further in a quantitative manner.
In 2001, we initiated and partially supported a research study on this subject at North Carolina State University. Soon Martin Marietta Technologies joined the study. (This company is a derivative of the Martin Marietta Technologies (formerly Lockheed Martin) aerospace company, but they are on the ground in the limestone business and probably produce most of the limestone in the world.) . Our idea was whether, with their resources, a horticultural lime could be developed. Though, like peat, transport is a major factor in lime business, a lime produced differently for horticulture could have a good market.
Peat Dimensions
Longtime growers and industry specialists know that not all peats are the same. (At first when you joined the industry, you thought all peats looked the same didn’t you? But you soon realized they’re not all the same.)
Though all fall under the genus Sphagnum, there are several distinct species. Further, within each species peat can be at a different decomposition stage. Still more, one would expect that the water peat has been in contact with during its formation would influence its pH—groundwater or rainwater. All these differences can affect a peat’s inherent acidity that has to be neutralized. The question is, which of these characters predict a peat’s lime requirement?
During late 2002, nearly 500 peat samples were collected from different fields in Alberta bogs. In each sample, species were tediously separated and their percentages estimated. Their decomposition rate was also determined. The species content of Alberta peat varied. Why? A natural bog is not flat. For example, Alberta bogs naturally have hummock- hollow type topography and have major species occurring (figure 2).
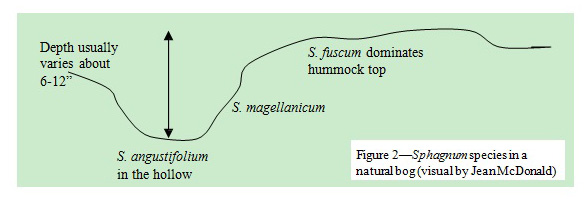 The differences in species correlate to differences in pH. Sphagnum fuscum on top of the hummock generally has a pH of 3.5. This species is horticulturally the best peat, because it has high water absorption capacity and decays slowly. Sphagnum megellanicum also absorbs water fast and retains it well, but it decomposes quickly. Sphagnum angustifolium, which resides in the hollows, has a pH of 4 to 5. There are also non-Sphagnums like sedges or debris, which not only have higher pHs but also increase pH variability.
The differences in species correlate to differences in pH. Sphagnum fuscum on top of the hummock generally has a pH of 3.5. This species is horticulturally the best peat, because it has high water absorption capacity and decays slowly. Sphagnum megellanicum also absorbs water fast and retains it well, but it decomposes quickly. Sphagnum angustifolium, which resides in the hollows, has a pH of 4 to 5. There are also non-Sphagnums like sedges or debris, which not only have higher pHs but also increase pH variability.
Why are there pH differences between Sphagnum species? All Sphagnum species have sites loaded with exchangeable hydrogen (acid) ions but the number of sites and the percent that are already saturated with bases vary. The saturation is influenced by how the species existed with respect to the water table. Sphagnum angustifolium, which is in the hollows, derives its moisture from water that is in contact with mineral soil. Such water has bases like calcium, magnesium, sodium, etc., which exchange places with hydrogen on peat. Therefore, S. angustifolium has a higher percent of its sites saturated with bases— so less hydrogen or acid ions and a higher pH. Sphagnum fuscum,which is on top of the hummock, derives its moisture from precipitation only and there are few bases in rain water. Therefore, S. fuscum still has more hydrogen or acid ions and a lower pH.
 What happens to pH as peat decomposes? Peats that are highly decomposed have high pH. When the naturally undulating bog surface is made flat to allow for harvesting, each pass of harvester picks up variation in the species and decomposition, resulting in variation in peat pH (see figures 3a and 3b).
What happens to pH as peat decomposes? Peats that are highly decomposed have high pH. When the naturally undulating bog surface is made flat to allow for harvesting, each pass of harvester picks up variation in the species and decomposition, resulting in variation in peat pH (see figures 3a and 3b).
Further variation occurs over time as well. Because a field that has been harvested for a long time would have a different mix of species and different decomposition stages than a newly opened field. As an example, species content in different fields in a sample location are shown in figure 4. Such diagrams drawn for different fields can be used to know the homogeneity of peat and therefore predictability of pH variation.
You might expect a peat starting at a pH of 4.5 to reach a target of 5.8 with less lime than a peat starting at a pH of 3.5. You would think in the peat with pH 4.5, some acidity would be already reduced by bases, making neutralization requirement lower. There is some relation between higher starting pH and lower lime requirement but the relation is not strong (just 20%). Why? A peat with a pH of 4.5 can still have a much higher number of sites available for bases than a peat with a pH of 3.5.
 Confusing? Let me explain with an analogy of a hotel versus a small motel. Until you know the total capacity of each lodging, knowing there are 25 rooms occupied in each lodging gives you little information about how many rooms are still vacant. The pH value of each peat is comparable. Even when bases like calcium and magnesium are extracted, a peat sample can yield a greater quantity of bases (equal to a greater number of guests) and still have a lower pH (equal to a lower percent occupancy).
Confusing? Let me explain with an analogy of a hotel versus a small motel. Until you know the total capacity of each lodging, knowing there are 25 rooms occupied in each lodging gives you little information about how many rooms are still vacant. The pH value of each peat is comparable. Even when bases like calcium and magnesium are extracted, a peat sample can yield a greater quantity of bases (equal to a greater number of guests) and still have a lower pH (equal to a lower percent occupancy).
To predict the lime requirement of a peat, one must determine the total capacity for base saturation, and the percent of that capacity already saturated with bases. The correlation between increasing percent of base saturation and decreasing lime requirement is stronger (40%). The total capacity of a peat to hold bases is based on its cation exchange capacity, where cations (positively charged ions like hydrogen, calcium, magnesium) are swapped for one another.
A high cation exchange capacity generally imparts a high buffer capacity. During growing, a peat with high buffer capacity will have great ability to trade other cations for hydrogen ions that are coming from plants, fertilizers, microbial action, etc. Thus, that kind of peat resists pH drifts, which is a property we desire in a mix. Sphagnum fuscum seems to be a species with high buffer capacity.
Finally, we generally think hydrogen is the source of acidity in peat. But interestingly, iron is found in Alberta peat. Iron bonds with hydroxide in water leaving acidic hydrogen ions in the solution. Variations in peat pH could be due to differing amounts of iron as well.
Lime Dimensions
Lime costs <1% of the mix price. But the value and the headaches it brings are well known. Most lime companies primarily serve the construction market and to them agricultural lime is a byproduct. Consequently lime companies don’t have technical people knowledgeable of our industry. The burden is on us to decide whether their lime serves our purpose or not.
Lime has to dissolve to act and neutralize peat acidity. We generally agree that the size of a lime particle affects its solubility: smaller particles dissolve faster. That’s how agriculture lime is characterized now. Unfortunately, this is yet another time of getting into trouble when agriculture perceptions are transferred to horticulture. In agronomy, they have time. A slow rise in soil pH in 3-4 months and a residual lime effect that lasts for 3 years is okay in field crops. In greenhouse growing, we want rapid rise— in days if not hours— and the residual effect for just 3-4 months. Our industry experience has been that the same amount of lime with the same sieve analysis (equal particle size) doesn’t always give the same pH in mixes. This result makes one suspect that particle size alone may not characterize horticultural lime sufficiently.
 Nine lime samples from our production plants (figure 5) and 40 from Martin Marietta quarries were gathered. Among these limes, there were dolomites (comprise calcium and magnesium carbonates) and calcites (comprise just calcium carbonate). These samples were tested.
Nine lime samples from our production plants (figure 5) and 40 from Martin Marietta quarries were gathered. Among these limes, there were dolomites (comprise calcium and magnesium carbonates) and calcites (comprise just calcium carbonate). These samples were tested.
When these limes were tested to find out how fast they react and neutralize acid, there were tremendous variations in dolomites coming from different sources. But there was very little or no variation between calcitic limes (indicating it is difficult to change dolomite sources and easier to change calcite sources).
Smaller lime particles did react faster than larger particles, but the tremendous variations in reaction rates were not fully related to particle size alone. Particle size accounted for only half of the reaction rate of lime. This finding gives an explanation of how mix pH can vary despite using limes having the same sieve analysis, if these limes are coming from different sources.
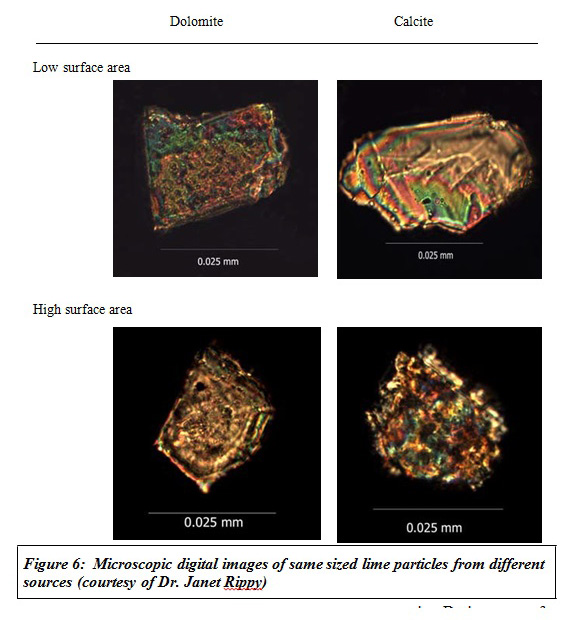 Same sized lime particles taken from different sources were reacted. Dolomites took longer time to react than calcites— four times longer on average. So, is reaction slower because of the presence of magnesium carbonate in dolomite? When limes were listed in the order of increasing magnesium carbonate content (which is the same as decreasing calcium carbonate content), magnesium carbonate content showed an influence on slowing the reaction rate, but again the reaction rate was not fully related to magnesium/calcium content.
Same sized lime particles taken from different sources were reacted. Dolomites took longer time to react than calcites— four times longer on average. So, is reaction slower because of the presence of magnesium carbonate in dolomite? When limes were listed in the order of increasing magnesium carbonate content (which is the same as decreasing calcium carbonate content), magnesium carbonate content showed an influence on slowing the reaction rate, but again the reaction rate was not fully related to magnesium/calcium content.
What other character of lime is influencing its neutralization capacity? Surface area of lime particles was measured. Same size lime particles from different sources had different surface areas— up to a 5-fold difference. This indicates that the exposed area on some lime particles is not just on the geometric surface and that there is considerable internal surface.
So far, particle size, content, surface area account for 80% of the neutralization capacity of lime. These findings tell us that in addition to the sieve analysis, and of course reaction rate, content and surface area should be included in the specs to further reduce the variability of lime.
More data on other lime characters is being analyzed to account for 100% of the neutralization capacity.
Matching Peat-lime
The goal of the research is to find out which characters determine neutralization capacity of lime and which characters determine neutralization requirement of peat and then match them suitably. (Like what the eHarmony guy says about profiling different dimensions of men and women before matching soul mates!)
Continuing with the matchmaking analogy, you might be wondering: What about the chemistry after marriage— pH drift during growing? The properties mentioned above give information to predict pH changes during growing as well. For example, different reaction rates of limes give information on balancing initial pH of the mix with pH maintenance during its use. For simplicity, I presented here the effect of one factor at a time, as if other factors remain the same. In the real world, as you can imagine, the peat-lime reactions occur in multifaceted dimensions in space and time.
But since the whole effect is a sum of its parts, depending on the situation, we can select and add or subtract relevant factors and evaluate the effect.
Research & Further Research
People in the industry should understand that large randomized, controlled research trials like this one are slow and frustrating at times. This study began in 2001, and conclusions after going through the vigilance of peer review are just coming out. But imagine 500 peat samples, 50 lime samples sieved into 8 fractions, each sample studied in replications for many different characters.
Just devising a test on how to measure a character took time. For example, how to measure the surface area of lime particle (dinitrogen gas was used as absorbate because the gas accesses the same areas of the particle as water does), what type of base to use to disassociate hydrogen ions from peat, etc.
Still, we should support research. Just on the subject of pH, we know pH brought on contentions, disputes and even litigations between people and between companies. During some of those times, following the explanations and remedies suggested by well meaning participants was like following the moves of mouse in a maze, due to lack of good information.
Working in the field we especially know how lack of good information adds uncertainty to our interpretation of a situation. New insights from research will reduce the uncertainty.
The research initiated by Sun Gro, NC State University and Martin Marietta branched into different angles. In 2004, Dr. Paul Fisher of the University of New Hampshire, got the remains of the large collection of Alberta peat samples to find out whether plants can mine the iron in some of those peats (like people do in Caribou Mountains in Alberta). His research is supported by a consortium of grower organizations and companies.
USDA funded Professor Paul Nelson to find out why some crops like geraniums push the mix pH down. Apparently, the protein that transports nitrate in geranium roots is somehow inhibited which leads to geranium taking up nitrogen in the form of ammonium, which causes low pH in the mix.
All these studies will let us position the pieces properly in the pH jigsaw puzzle and ultimately result in great looking plants like the one in figure 7 all the time.
Listed below are 4 research papers from this study that have been published so far and more are forthcoming. The papers have detailed work for interested people:
1) “Specific surface versus particle diameter of limestones” by Janet Rippy et al. Presented at the American Society of Horticultural Science Conference in Austin, Texas on 19 July 2004.
2) “Soilless root substrate pH meas- urement technique for titration” by Janet Rippy and Paul Nelson, pub- lished in HortScience, February 2005.
3) “Lime specific surface versus particle size & Reaction times of twenty limestones” by Janet Rippy et al., to be published in Communications in Soil Science and Plant Analysis.
Janet Rippy and Professor Paul Nelson were the chief researchers. Former resident peat expert at Seba Beach, Tony Cable and botanist from Alberta, Jean McDonald helped in peat sampling and identification.
Bryology Professor Dale Vitt from Southern Illinois University Carbondale participated in the peat background discussions. David Jahn from Martin Marietta helped in lime sampling, analyses and discussions. Tech specialists from all the regions helped in getting lime samples. Mark Spong supported the study.
~Shiv Reddy
